Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Editorial
Catalysis and Sustainable Chemistry
Adam F. Lee
Centre for Catalysis and Clean Energy, Griffith University, Gold Coast, QLD 4222, Australia; adam.lee@griffith.edu.au
Received: 4 November 2024; Accepted: 5 November 2024; Published: 13 November 2024
Chemical manufacturing is critical for achieving sustainable development and a high quality of life for the global population. It underpins 95% of all manufactured goods and contributes US$5.7 trillion and 150 million jobs to the global economy (excluding refined fossil fuels) [1]. However, the chemical industry is also the world’s largest industrial energy consumer, third-largest producer of direct CO2 emissions, and has inadvertently facilitated the release of toxic and persistent chemicals into the Earth’s ecosystem [2]. For example, the Australian chemical industry is the country’s second-largest manufacturing sector, underpinning ~218,000 full-time employees and revenues of ~US$37B in 2018 [3], yet relies heavily on fossil gas as a feedstock and imports for fuels and almost all healthcare and consumer products (Figure 1) [3]. These imports are overwhelmingly synthesised from organic molecules derived from fossil hydrocarbons that negatively impact the environment. In parallel, Australia generates vast quantities of organic carbon waste [4] which accounts for ~20% of national CO2 emissions and ~$69B of lost value (Figure 1) [5–8]. Sustainable manufacturing of chemical building blocks from onshore renewable (waste) carbon sources offers nations sovereign capability, the opportunity to mitigate climate change and environmental pollution through new circular economies, and derisking the impact of severe climate events.
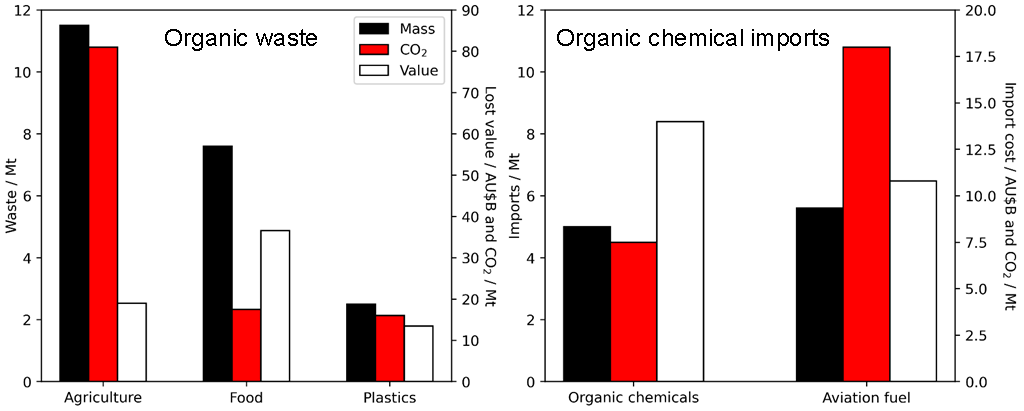
Figure 1. (left) Approximate renewable carbon resources available for chemical manufacturing within Australia, and (right) externally sourced carbon imported to Australia, data compiled from [3–5].
In contrast to the energy sector, which has witnessed rapid adoption of renewable generating and storage technologies in recent decades, the chemical industry has remained reliant on fossil feedstocks, a small number of chemical commodities, massive economies of scale, rigid manufacturing processes, and limited innovation [2]. Petrochemicals and their derivatives are highly integrated into today’s fossil-fuel economy, being tied to sunk investments, complex global supply chains, and rigid infrastructure. Transitioning to carbon-neutral feedstocks while relying on existing manufacturing processes will fail to address waste and toxicity issues and disproportionate impacts on vulnerable communities; a transformed chemical industry must also reduce resource consumption (through a circular economy) and promote a nontoxic environment. Achieving an industry transition of the requisite magnitude required necessitates an entire rethink of the centralised and capital-intensive technologies employed in today’s chemical manufacturing [2,6,9–10].
Catalysts are substances that lower the energy barriers for chemical transformations, while themselves being regenerated after each reaction cycle, and are a key enabling technology across the chemical industry [11–13]. However, despite a rich 150-year history of catalyst development, many commercial chemical processes are the result of serendipity or laborious trial-and-error. This reflects the complex interdependent interactions between energy and matter that dictate the outcome of catalysis. Understanding the fundamental processes involved in the transport of molecules and energy to and from a catalyst requires a multifaceted approach encompassing materials science, thermodynamics, kinetics, and reaction engineering [14]. Almost all modern chemical manufacturing depends on the catalytic transformation of a narrow range of fossil feedstocks, often at high temperatures in non-aqueous environments. Entirely new catalytic strategies to exploit renewable feedstocks, such as biomass, into products with unique performance and amenable to recycling at their end of life are critical for compliance with 2030 UN Sustainable Development Goals [15], notably: Goal 9 (“upgrade all industries and infrastructures for sustainability; enhance research and upgrade industrial technologies”); Goal 12 (“reducing by half the per capita global food waste; environmentally sound management of chemicals and all wastes throughout their life cycle; reducing waste generation through prevention, reduction, recycling and reuse”); and Goal 14 (“reducing impacts from marine plastic pollution”). Chemical manufacturing from renewable sources of carbon, such as lignocellulosic biomass powered by photosynthesis or atmospheric CO2 released by anthropogenic activities, would mark a new era in the Holocene, unlocking the possibility of reversing negative human impact on the biosphere. Solving this grand challenge requires concerted multidisciplinary efforts from chemists, engineers, materials scientists, and environmental and social scientists.
The past decade has seen a transition from the large-scale use of seed oils (that compete with food sources) to second-generation energy crops (that compete for land use) and finally to whole biomass, as renewable feedstocks for chemicals and fuels. This transition has been enabled by advances in: (i) biomass processing; (ii) catalyst design; and (iii) reactor technologies. Biomass fractionation is widely used to extract the carbohydrate component but co-produces an unusable lignin component. So-called “lignin first” approaches [16] have unlocked access to sugar derivatives as so-called “platform chemicals”, molecular building blocks for plastics, solvents, coatings, surfactants and lubricants, and a parallel stream of phenolics, molecular building blocks for fuels and pharmaceuticals. New strategies for the bottom-up [17] or top-down fabrication of catalysts provide unprecedented control over the placement of chemically active atoms and their surroundings [18]. This permits the replacement of toxic and hazardous reagents or soluble (homogeneous) catalysts that cannot be easily separated from desired products. Powerful computational methods, including artificial intelligence (AI), now allow the chemical reactivity of catalysts to be predicted with reasonable accuracy [19], even for large molecules in complex environments (solvents and electrolytes). Sophisticated analytical tools, such as electron microscopy [20] and X-ray spectroscopy, can now be applied to interrogate working catalysts in real-time (termed “in operando”) to validate computational models and guide catalyst formulation. Finally, additive manufacturing and the uptake of flow reactors facilitate the manufacture of bespoke, complex reactor geometries that improve reactant mixing and product separation [21], and the continuous optimisation of chemical reactions with improved performance, safety, and energy efficiency. The advent of compact, modular reactors will afford small-scale distributed chemical synthesis proximate to waste resources, circumventing the need for capital-intensive, centralised chemical plants and the associated tyranny of transport.
The journal Catalysis and Sustainable Chemistry offers a forum for academic and industry practitioners, and stakeholders responsible for formulating and implementing sustainability policy, to disseminate scientific breakthroughs and innovative proposals to advance sustainable chemical manufacturing.
Conflicts of Interest: The author declares no conflict of interest.
References
- Catlow, C.R; Davidson, M.; Hardacre, C.; Hutchings, G.J. Catalysis making the world a better place. Phil. Trans. R. Soc. A 2016, 374, 20150089.
- Tickner, J.A.; Geiser, K.; Baima, S. Transitioning the chemical industry: elements of a roadmap toward sustainable chemicals and materials. Environ. Sci. Policy Sustain. Dev. 2022, 64, 22–36.
- ACIL Allen Consulting, Chemical Industry Economic Contribution Analysis, 2017-18. 2019. Available online: https://acilallen.com.au/uploads/projects/168/ACILAllen_ChemicalIndustry2019-1565671864.pdf (accessed on 13 November 2024).
- World Integrated Trade Solution. 2024. Available online: https://wits.worldbank.org/CountryProfile/en/Country/AUS/Year/LTST/TradeFlow/Import/Partner/by-country/Product/28-38_Chemicals (accessed on 13 November 2024).
- Pickin, J.; Wardle, C.; O’Farrell, K.; Stovell, L.; Nyunt, P.; Guazzo, S.; Lin, Y.; Caggiati-Shortell, G.; Chakma, P.; Edwards, C.; et al. National Waste Report; Blue Environment Pty Ltd.: Docklands, VIC, Australia, 2022.
- Our Ambition for Australia—Opportunities for a Sustainable and Competitive Economy, Chemistry Australia Policy Priorities White Paper, 2022. Available online: https://chemistryaustralia.org.au/images/chemaust/policy/Chemistry-Australia-Policy-Priorities-2022.pdf (accessed on 4 November 2024).
- Australian National Greenhouse Accounts Factors Workbook 2022, Department of Climate Change, Energy, the Environment and Water. Available online: https://www.dcceew.gov.au/sites/default/files/documents/national-greenhouse-accounts-factors-2022.pdf (accessed on 4 November 2024).
- Costs and Benefits of Banning Exports of Waste, 2020, The Centre for International Economics. Available online: https://www.dcceew.gov.au/sites/default/files/documents/costs-benefits-banning-exports-waste.pdf (accessed on 4 November 2024).
- Available online: https://www.weforum.org/stories/2022/11/chemical-industry-fossil-fuels-decarbonization/ (accessed on 4 November 2024)
- Available online: https://www.weforum.org/stories/2024/01/how-next-decade-define-transformation-chemical-industry/ (accessed on 4 November 2024).
- Mitchell, S.; Martín, A.J.; Pérez-Ramírez, J. Transcending Scales in Catalysis for Sustainable Development. Nat. Chem. Eng. 2024, 1, 13–15.
- Abbas, A.; Cross, M.; Duan, X.; Jeschke, S.; Konarova, M.; Huber, G.W.; Lee, A.F.; Lovell, E.C.; Lim, J.Y.C.; Polyzos, A.; et al. Catalysis at the Intersection of Sustainable Chemistry and a Circular Economy. One Earth 2024, 7, 738–741. 10.1016/j.oneear.2024.04.018
- Taseska, T.; Yu, W.; Wilsey, M.K.; Cox, C.P.; Meng, Z.; Ngarnim, S.S.; Müller, A.M. Analysis of the Scale of Global Human Needs and Opportunities for Sustainable Catalytic Technologies. Top. Catal. 2023, 66, 338–374.
- Bollini, P.; Diwan, M.; Gautam, P.; Hartman, R.L.; Hickman, D.A.; Johnson, M.; Kawase, M.; Neurock, M.; Patience, G.S.; Stottlemyer, A.; et al. Vision 2050: Reaction Engineering Roadmap. ACS Eng. Au 2023, 3, 364–390.
- United Nations 2030 Agenda for Sustainable Development, Department of Economic and Social Affairs. Available online: https://sdgs.un.org/goals (accessed on 13 November 2024).
- Renders, T; Van den Bosch, S.; Koelewijn, S.-F.; Schutyser, W.; Sels, B.F. Lignin-First Biomass Fractionation: The Advent of Active Stabilisation Strategies. Energy Environ. Sci. 2017, 10, 1551–1557.
- Yan, H.; Lin, Y.; Wu, H.; Zhang, W.; Sun, Z.; Cheng, H.; Liu, W.; Wang, C.; Li, J.; Huang, X.; et al. Bottom-Up Precise Synthesis of Stable Platinum Dimers on Graphene. Nat. Commun. 2017, 8, 1070.
- Xing, L.; Jin, Y; Weng, Y.; Feng, R.; Ji, Y.; Gao, H.; Chen, X.; Zhang, X.; Jia, D.; Wang, G. Top-Down Synthetic Strategies toward Single Atoms on the Rise. Matter 2022, 5, 788–807.
- Mou, T.; Pillai, H.S.; Wang, S.; Wan, M.; Han, X.; Schweitzer, N.M.; Che, F.; Xin, H. Bridging the Complexity Gap in Computational Heterogeneous Catalysis with Machine Learning. Nat. Catal. 2023, 6, 122–136.
- Wu, H.; Zhao, X.; Song, D.; Tian, F.; Wang, J.; Loh, K.P.; Pennycook, S.J. Progress and Prospects of Aberration-Corrected STEM for Functional Materials. Ultramicroscopy 2018, 194, 182–192.
- Zhu, J.; Wu, P.; Chao, Y.; Yu, J.; Zhu, W.; Liu, Z.; Xu, C. Recent Advances in 3D Printing for Catalytic Applications. Chem. Eng. J. 2022, 433, 134341.





