Downloads
Download


This work is licensed under a Creative Commons Attribution 4.0 International License.
Review
From Clinical to Basic Research: The Neuroprotective Effects and Mechanisms of Caffeine
Lijing Wang and Linxi Wang *
Department of Endocrinology and Metabolism, Fujian Institute of Endocrinology, Fujian Medical University Union Hospital, Fuzhou 350001, China
* Correspondence: wanglinxi@fjmu.edu.cn
Received: 15 October 2024; Revised: 31 October 2024; Accepted: 21 February 2025; Published: 1 April 2025
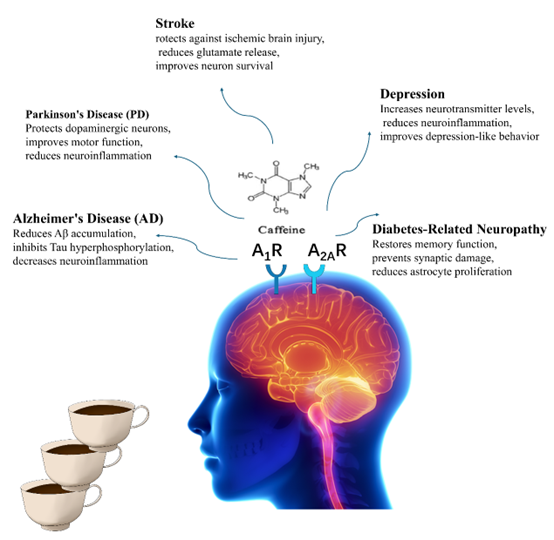
Abstract: Caffeine is the most widely used psychoactive substance in the world, is present in various beverages such as coffee, tea, and energy drinks. Its basic chemical structure contains methylxanthine active components. As a non-selective central adenosine receptor antagonist, caffeine exerts a broad range of pharmacological effects, including antioxidant, anti-inflammatory, and neuroprotective functions. Epidemiological studies and clinical reports suggest that caffeine consumption is closely associated with a reduced risk of neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, and dementia. Additionally, caffeine has shown potential benefits in regulating cognitive function, improving depressive symptoms, and reducing the risk of stroke. Although the neuroprotective mechanisms of caffeine remain unclear, current research has revealed that it exerts its effects through multiple signaling pathways, including the inhibition of adenosine A2A receptors, the suppression of neuroinflammation, and the modulation of synaptic plasticity. This paper discusses the recent advancements in research on the neuroprotective effects of caffeine and explores its potential mechanisms and applications in Alzheimer’s disease, Parkinson’s disease, stroke, and depression.
Keywords:
caffeine Alzheimer’s disease Parkinson’s disease stroke depressionReferences
- Drewnowski, A.; Rehm, C.D. Sources of Caffeine in Diets of US Children and Adults: Trends by Beverage Type and Purchase Location. Nutrients 2016, 8, 154. https://doi.org/10.3390/nu8030154.
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. https://doi.org/10.1056/NEJMra1816604.
- Marcus, C.L.; Meltzer, L.J.; Roberts, R.S.; Traylor, J.; Dix, J.; D’Ilario, J.; Asztalos, E.; Opie, G.; Doyle, L.W.; Biggs, S.N.; et al. Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am. J. Respir. Crit. Care Med. 2014, 190, 791–799. https://doi.org/10.1164/rccm.201406-1092OC.
- Derry, C.J.; Derry, S.; Moore, R.A. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst. Rev. 2014, 2014, CD009281. https://doi.org/10.1002/14651858.CD009281.pub3.
- Gokcen, B.B.; Sanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. https://doi.org/10.1080/10408398.2017.1369391.
- Oleaga, C.; Ciudad, C.J.; Noe, V.; Izquierdo-Pulido, M. Coffee polyphenols change the expression of STAT5B and ATF-2 modifying cyclin D1 levels in cancer cells. Oxid. Med. Cell. Longev. 2012, 2012, 390385. https://doi.org/10.1155/2012/390385.
- Santos, R.M.; Lima, D.R. Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur. J. Nutr. 2016, 55, 1345–1358. https://doi.org/10.1007/s00394-016-1206-0.
- Homan, D.J.; Mobarhan, S. Coffee: Good, bad, or just fun? A critical review of coffee’s effects on liver enzymes. Nutr. Rev. 2006, 64, 43–46. https://doi.org/10.1111/j.1753-4887.2006.tb00172.x.
- Shan, L.; Zhao, N.; Wang, F.; Zhai, D.; Liu, J.; Lv, X. Caffeine in Hepatocellular Carcinoma: Cellular Assays, Animal Experiments, and Epidemiological Investigation. J. Inflamm. Res. 2024, 17, 1589–1605. https://doi.org/10.2147/jir.S424384.
- Arendash, G.W.; Cao, C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S117–S126. https://doi.org/10.3233/JAD-2010-091249.
- Mirzaei, F.; Agbaria, L.; Bhatnagar, K.; Sirimanne, N.; Omar A’amar, N.; Jindal, V.; Gerald Thilagendra, A.; Tawfiq Raba, F. Coffee and Alzheimer’s disease. Prog. Brain Res. 2024, 289, 21–55. https://doi.org/10.1016/bs.pbr.2024.06.002.
- Trevitt, J.; Kawa, K.; Jalali, A.; Larsen, C. Differential effects of adenosine antagonists in two models of parkinsonian tremor. Pharmacol. Biochem. Behav. 2009, 94, 24–29. https://doi.org/10.1016/j.pbb.2009.07.001.
- de Mendonca, A.; Cunha, R.A. Therapeutic opportunities for caffeine in Alzheimer’s disease and other neurodegenerative disorders. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S1–S2. https://doi.org/10.3233/JAD-2010-01420.
- Evans, J.; Richards, J.R.; Battisti, A.S. Caffeine; StatPearls: Treasure Island, FL, USA, 2024.
- Bonati, M.; Latini, R.; Tognoni, G.; Young, J.F.; Garattini, S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat, and mouse. Drug Metab. Rev. 1984, 15, 1355–1383. https://doi.org/10.3109/03602538409029964.
- Tanaka, H.; Nakazawa, K.; Arima, M.; Iwasaki, S. Caffeine and its dimethylxanthines and fetal cerebral development in rat. Brain Dev. 1984, 6, 355–361. https://doi.org/10.1016/s0387-7604(84)80111-4.
- Rybak, M.E.; Pao, C.I.; Pfeiffer, C.M. Determination of urine caffeine and its metabolites by use of high-performance liquid chromatography-tandem mass spectrometry: Estimating dietary caffeine exposure and metabolic phenotyping in population studies. Anal. Bioanal. Chem. 2014, 406, 771–784. https://doi.org/10.1007/s00216-013-7506-9.
- Guo, J.; Zhu, X.; Badawy, S.; Ihsan, A.; Liu, Z.; Xie, C.; Wang, X. Metabolism and Mechanism of Human Cytochrome P450 Enzyme 1A2. Curr. Drug Metab. 2021, 22, 40–49. https://doi.org/10.2174/1389200221999210101233135.
- Abu‐Hashem, A.A.; Hakami, O.; El‐Shazly, M.; El‐Nashar, H.A.; Yousif, M.N. Caffeine and Purine Derivatives: A Comprehensive Review on the Chemistry, Biosynthetic Pathways, Synthesis-Related Reactions, Biomedical Prospectives and Clinical Applications. Chem. Biodivers. 2024, 21, e202400050. https://doi.org/10.1002/cbdv.202400050.
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133.
- Ribeiro, J.; Sebastião, A. Caffeine and adenosine. J. Alzheimer Dis. 2010, 20, S3–S15. https://doi.org/10.3233/jad-2010-1379.
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. https://doi.org/10.3389/fphar.2017.00661.
- Fredholm, B.B.; Chen, J.F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.M. Adenosine and brain function. Int. Rev. Neurobiol. 2005, 63, 191–270. https://doi.org/10.1016/S0074-7742(05)63007-3.
- Fredholm, B.B.; AP, I.J.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552.
- Karcz-Kubicha, M.; Antoniou, K.; Terasmaa, A.; Quarta, D.; Solinas, M.; Justinova, Z.; Pezzola, A.; Reggio, R.; Muller, C.E.; Fuxe, K.; et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 2003, 28, 1281–1291. https://doi.org/10.1038/sj.npp.1300167.
- Mohamed, R.A.; Agha, A.M.; Abdel-Rahman, A.A.; Nassar, N.N. Role of adenosine A2A receptor in cerebral ischemia reperfusion injury: Signaling to phosphorylated extracellular signal-regulated protein kinase (pERK1/2). Neuroscience 2016, 314, 145–159. https://doi.org/10.1016/j.neuroscience.2015.11.059.
- Rombo, D.M.; Newton, K.; Nissen, W.; Badurek, S.; Horn, J.M.; Minichiello, L.; Jefferys, J.G.; Sebastiao, A.M.; Lamsa, K.P. Synaptic mechanisms of adenosine A2A receptor-mediated hyperexcitability in the hippocampus. Hippocampus 2015, 25, 566–580. https://doi.org/10.1002/hipo.22392.
- Sitkovsky, M.V.; Lukashev, D.; Apasov, S.; Kojima, H.; Koshiba, M.; Caldwell, C.; Ohta, A.; Thiel, M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004, 22, 657–682. https://doi.org/10.1146/annurev.immunol.22.012703.104731.
- Hu, Q.; Ren, X.; Liu, Y.; Li, Z.; Zhang, L.; Chen, X.; He, C.; Chen, J.F. Aberrant adenosine A2A receptor signaling contributes to neurodegeneration and cognitive impairments in a mouse model of synucleinopathy. Exp. Neurol. 2016, 283, 213–223. https://doi.org/10.1016/j.expneurol.2016.05.040.
- Li, P.; Rial, D.; Canas, P.M.; Yoo, J.H.; Li, W.; Zhou, X.; Wang, Y.; van Westen, G.J.; Payen, M.P.; Augusto, E.; et al. Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol. Psychiatry 2015, 20, 1481. https://doi.org/10.1038/mp.2015.43.
- Prasanthi, J.R.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. https://doi.org/10.1016/j.freeradbiomed.2010.07.007.
- Alkanad, M.; Hani, U.; Annegowda, H.V.; Ghazwani, M.; Haider, N.; Osmani, R.; Pandareesh, M.D.; Hamsalakshmi; Bhat, R. Bitter yet beneficial: The dual role of dietary alkaloids in managing diabetes and enhancing cognitive function. BioFactors 2024, 50, 634–673. https://doi.org/10.1002/biof.2034.
- Maia, L.; de Mendonca, A. Does caffeine intake protect from Alzheimer’s disease? Eur. J. Neurol. 2002, 9, 377–382. https://doi.org/10.1046/j.1468-1331.2002.00421.x.
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife coffee and tea drinking and the risk of late-life dementia: A population-based CAIDE study. J. Alzheimers Dis. 2009, 16, 85–91. https://doi.org/10.3233/JAD-2009-0920.
- Ritchie, K.; Carriere, I.; de Mendonca, A.; Portet, F.; Dartigues, J.F.; Rouaud, O.; Barberger-Gateau, P.; Ancelin, M.L. The neuroprotective effects of caffeine: A prospective population study (the Three City Study). Neurology 2007, 69, 536–545. https://doi.org/10.1212/01.wnl.0000266670.35219.0c.
- Larsson, S.C.; Woolf, B.; Gill, D. Plasma Caffeine Levels and Risk of Alzheimer’s Disease and Parkinson’s Disease: Mendelian Randomization Study. Nutrients 2022, 14, 1697. https://doi.org/10.3390/nu14091697.
- Bateman, R.J.; Aisen, P.S.; De Strooper, B.; Fox, N.C.; Lemere, C.A.; Ringman, J.M.; Salloway, S.; Sperling, R.A.; Windisch, M.; Xiong, C. Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 2011, 3, 1. https://doi.org/10.1186/alzrt59.
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245. https://doi.org/10.1016/j.expneurol.2006.08.008.
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tane, J.; Citron, B.A.; Lin, X.; et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J. Alzheimers Dis. 2009, 17, 661–680. https://doi.org/10.3233/JAD-2009-1087.
- Giunta, S.; Andriolo, V.; Castorina, A. Dual blockade of the A1 and A2A adenosine receptor prevents amyloid beta toxicity in neuroblastoma cells exposed to aluminum chloride. Int. J. Biochem. Cell Biol. 2014, 54, 122–136. https://doi.org/10.1016/j.biocel.2014.07.009.
- Laurent, C.; Eddarkaoui, S.; Derisbourg, M.; Leboucher, A.; Demeyer, D.; Carrier, S.; Schneider, M.; Hamdane, M.; Muller, C.E.; Buee, L.; et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol. Aging 2014, 35, 2079–2090. https://doi.org/10.1016/j.neurobiolaging.2014.03.027.
- M Yelanchezian, Y.M.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Neuroprotective Effect of Caffeine in Alzheimer’s Disease. Molecules 2022, 27, 3737. https://doi.org/10.3390/molecules27123737.
- Leite, M.R.; Wilhelm, E.A.; Jesse, C.R.; Brandao, R.; Nogueira, C.W. Protective effect of caffeine and a selective A2A receptor antagonist on impairment of memory and oxidative stress of aged rats. Exp. Gerontol. 2011, 46, 309–315. https://doi.org/10.1016/j.exger.2010.11.034.
- Ullah, F.; Ali, T.; Ullah, N.; Kim, M.O. Caffeine prevents d-galactose-induced cognitive deficits, oxidative stress, neuroinflammation and neurodegeneration in the adult rat brain. Neurochem. Int. 2015, 90, 114–124. https://doi.org/10.1016/j.neuint.2015.07.001.
- Wostyn, P.; Van Dam, D.; Audenaert, K.; De Deyn, P.P. Increased Cerebrospinal Fluid Production as a Possible Mechanism Underlying Caffeine’s Protective Effect against Alzheimer’s Disease. Int. J. Alzheimers Dis. 2011, 2011, 617420. https://doi.org/10.4061/2011/617420.
- Ren, X.; Chen, J.F. Caffeine and Parkinson’s Disease: Multiple Benefits and Emerging Mechanisms. Front. Neurosci. 2021, 14, 602697. https://doi.org/10.3389/fnins.2020.602697.
- Suraj, R.; Bonnie, K. Parkinson’s Disease: Risk Factor Modification and Prevention. Semin. Neurol. 2022, 42, 626–638. https://doi.org/10.1055/s-0042-1758780.
- Heinz, R.; Ilona, C.; Jiri, K.; Stefan, L.; Christoph, S.; Juergen, W.; Ullrich, W. Life style and Parkinson’s disease. J. Neural Transm. 2022, 129, 1235–1245. https://doi.org/10.1007/s00702-022-02509-1.
- Qi, H.; Li, S. Dose-response meta-analysis on coffee, tea and caffeine consumption with risk of Parkinson’s disease. Geriatr. Gerontol. Int. 2014, 14, 430–439. https://doi.org/10.1111/ggi.12123.
- Palacios, N.; Gao, X.; McCullough, M.L.; Schwarzschild, M.A.; Shah, R.; Gapstur, S.; Ascherio, A. Caffeine and risk of Parkinson’s disease in a large cohort of men and women. Mov. Disord. 2012, 27, 1276–1282. https://doi.org/10.1002/mds.25076.
- Altman, R.D.; Lang, A.E.; Postuma, R.B. Caffeine in Parkinson’s disease: A pilot open-label, dose-escalation study. Mov. Disord. 2011, 26, 2427–2431. https://doi.org/10.1002/mds.23873.
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Rios Romenets, S.; Altman, R.; et al. Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 2012, 79, 651–658. https://doi.org/10.1212/WNL.0b013e318263570d.
- Postuma, R.B.; Anang, J.; Pelletier, A.; Joseph, L.; Moscovich, M.; Grimes, D.; Furtado, S.; Munhoz, R.P.; Appel-Cresswell, S.; Moro, A.; et al. Caffeine as symptomatic treatment for Parkinson disease (Cafe-PD): A randomized trial. Neurology 2017, 89, 1795–1803. https://doi.org/10.1212/WNL.0000000000004568.
- Chen, J.F.; Schwarzschild, M.A. Do caffeine and more selective adenosine A(2A) receptor antagonists protect against dopaminergic neurodegeneration in Parkinson’s disease? Park. Relat. Disord. 2020, 80, S45–S53. https://doi.org/10.1016/j.parkreldis.2020.10.024.
- Heinz, R. Caffeine, Chocolate and Adenosine A2A Receptor Antagonists in the Treatment of Parkinson’s Disease. Fortschr. Neurol Psychiatr. 2022, 91, 3632. https://doi.org/10.1055/a-1785-3632.
- Xu, K.; Di Luca, D.G.; Orru, M.; Xu, Y.; Chen, J.F.; Schwarzschild, M.A. Neuroprotection by caffeine in the MPTP model of parkinson’s disease and its dependence on adenosine A2A receptors. Neuroscience 2016, 322, 129–137. https://doi.org/10.1016/j.neuroscience.2016.02.035.
- Bagga, P.; Chugani, A.N.; Patel, A.B. Neuroprotective effects of caffeine in MPTP model of Parkinson’s disease: A (13)C NMR study. Neurochem. Int. 2016, 92, 25–34. https://doi.org/10.1016/j.neuint.2015.11.006.
- Xu, K.; Xu, Y.; Brown-Jermyn, D.; Chen, J.F.; Ascherio, A.; Dluzen, D.E.; Schwarzschild, M.A. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neurosci. 2006, 26, 535–541. https://doi.org/10.1523/JNEUROSCI.3008-05.2006.
- Sonsalla, P.K.; Wong, L.Y.; Harris, S.L.; Richardson, J.R.; Khobahy, I.; Li, W.; Gadad, B.S.; German, D.C. Delayed caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson’s disease. Exp. Neurol. 2012, 234, 482–487. https://doi.org/10.1016/j.expneurol.2012.01.022.
- Xu, K.; Xu, Y.H.; Chen, J.F.; Schwarzschild, M.A. Neuroprotection by caffeine: Time course and role of its metabolites in the MPTP model of Parkinson’s disease. Neuroscience 2010, 167, 475–481. https://doi.org/10.1016/j.neuroscience.2010.02.020.
- Xu, K.; Bastia, E.; Schwarzschild, M. Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson’s disease. Pharmacol. Ther. 2005, 105, 267–310. https://doi.org/10.1016/j.pharmthera.2004.10.007.
- Machado-Filho, J.A.; Correia, A.O.; Montenegro, A.B.; Nobre, M.E.; Cerqueira, G.S.; Neves, K.R.; Naffah-Mazzacoratti Mda, G.; Cavalheiro, E.A.; de Castro Brito, G.A.; de Barros Viana, G.S. Caffeine neuroprotective effects on 6-OHDA-lesioned rats are mediated by several factors, including pro-inflammatory cytokines and histone deacetylase inhibitions. Behav. Brain Res. 2014, 264, 116–125. https://doi.org/10.1016/j.bbr.2014.01.051.
- Luan, Y.; Ren, X.; Zheng, W.; Zeng, Z.; Guo, Y.; Hou, Z.; Guo, W.; Chen, X.; Li, F.; Chen, J.F. Chronic Caffeine Treatment Protects Against α-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum. Front. Neurosci. 2018, 12, 301. https://doi.org/10.3389/fnins.2018.00301.
- Kachroo, A.; Schwarzschild, M. Adenosine A2A receptor gene disruption protects in an α-synuclein model of Parkinson’s disease. Ann. Neurol. 2012, 71, 278–282. https://doi.org/10.1002/ana.22630.
- Daphne, J.T.; Stavroula, J.T.; Andreas, Z.; Vasiliki, G.; Anna, G. Energy drinks enriched with caffeine and ischemic stroke. J. Clin. Neurosci. 2024, 12, 12–13. https://doi.org/10.1016/j.jocn.2023.12.016.
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2022, 32, 399–405.
- Zhang, W.; Lopez-Garcia, E.; Li, T.Y.; Hu, F.B.; van Dam, R.M. Coffee consumption and risk of cardiovascular diseases and all-cause mortality among men with type 2 diabetes. Diabetes Care 2009, 32, 1043–1045. https://doi.org/10.2337/dc08-2251.
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. https://doi.org/10.1161/CIRCULATIONAHA.113.005925.
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Rexrode, K.M.; Logroscino, G.; Hu, F.B.; van Dam, R.M. Coffee consumption and risk of stroke in women. Circulation 2009, 119, 1116–1123. https://doi.org/10.1161/CIRCULATIONAHA.108.826164.
- Piriyawat, P.; Labiche, L.; Burgin, W.; Aronowski, J.; Grotta, J. Pilot dose-escalation study of caffeine plus ethanol (caffeinol) in acute ischemic stroke. Stroke 2003, 34, 1242–1245. https://doi.org/10.1161/01.Str.0000067706.23777.04.
- Jinming, F.; Yajun, Y.; Xiaoting, Z.; Wenhan, L.; Wuqin, M.; Wenhao, W.; Jinyan, G.; Bin, Z. Association between urinary caffeine and caffeine metabolites and stroke in American adults: A cross-sectional study from the NHANES, 2009–2014. Sci. Rep. 2023, 13, 11855. https://doi.org/10.1038/s41598-023-39126-1.
- Gomes, C.V.; Kaster, M.P.; Tome, A.R.; Agostinho, P.M.; Cunha, R.A. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim. Biophys. Acta 2011, 1808, 1380–1399. https://doi.org/10.1016/j.bbamem.2010.12.001.
- Sutherland, G.R.; Peeling, J.; Lesiuk, H.J.; Brownstone, R.M.; Rydzy, M.; Saunders, J.K.; Geiger, J.D. The effects of caffeine on ischemic neuronal injury as determined by magnetic resonance imaging and histopathology. Neuroscience 1991, 42, 171–182. https://doi.org/10.1016/0306-4522(91)90157-j.
- Rudolphi, K.A.; Keil, M.; Fastbom, J.; Fredholm, B.B. Ischaemic damage in gerbil hippocampus is reduced following upregulation of adenosine (A1) receptors by caffeine treatment. Neurosci. Lett. 1989, 103, 275–280. https://doi.org/10.1016/0304-3940(89)90112-2.
- Potter, M.; Rosenkrantz, T.; Fitch, R.H. Behavioral and neuroanatomical outcomes in a rat model of preterm hypoxic-ischemic brain Injury: Effects of caffeine and hypothermia. Int. J. Dev. Neurosci. 2018, 70, 46–55. https://doi.org/10.1016/j.ijdevneu.2018.02.001.
- Evans, S.M.; Pinto Pereira, L.M.; Addae, J.I. Neuroprotection by caffeine and pentoxifylline during experimental cerebral ischaemia. West Indian Med. J. 1999, 48, 23–25.
- van Dam, R.M.; Hu, F.B. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA 2005, 294, 97–104. https://doi.org/10.1001/jama.294.1.97.
- Wang, L.; Ke, S.; Wang, L.; Huang, L.; Qi, L.; Zhan, Z.; Wu, K.; Zhang, M.; Liu, X.; Liu, X.; et al. Altered Caffeine Metabolism Is Associated With Recurrent Hypoglycemia in Type 2 Diabetes Mellitus: A UPLC-MS-Based Untargeted Metabolomics Study. Front. Endocrinol. 2022, 13, 843556. https://doi.org/10.3389/fendo.2022.843556.
- Gold, S.M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Rogers, K.; Bruehl, H.; Tsui, W.; Richardson, S.; Javier, E.; Convit, A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007, 50, 711–719. https://doi.org/10.1007/s00125-007-0602-7.
- Duarte, J.M.; Oliveira, C.R.; Ambrosio, A.F.; Cunha, R.A. Modification of adenosine A1 and A2A receptor density in the hippocampus of streptozotocin-induced diabetic rats. Neurochem. Int. 2006, 48, 144–150. https://doi.org/10.1016/j.neuint.2005.08.008.
- Gaspar, J.M.; Baptista, F.I.; Galvao, J.; Castilho, A.F.; Cunha, R.A.; Ambrosio, A.F. Diabetes differentially affects the content of exocytotic proteins in hippocampal and retinal nerve terminals. Neuroscience 2010, 169, 1589–1600. https://doi.org/10.1016/j.neuroscience.2010.06.021.
- Duarte, J.M.; Agostinho, P.M.; Carvalho, R.A.; Cunha, R.A. Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of NONcZNO10/LTJ mice. PLoS ONE 2012, 7, e21899. https://doi.org/10.1371/journal.pone.0021899.
- Gian Carlo, T.; Maria, D.; Valentina, O.; Emanuela, D.U.; Seyed Hassan, S.; Ettore, N.; Seyed Fazel, N.; Seyed Mohammad, N. Coffee and Depression: A Short Review of Literature. Curr. Pharm. Des. 2015, 21, 5034–5040. https://doi.org/10.2174/1381612821666150825145112.
- Park, R.J.; Moon, J.D. Coffee and depression in Korea: The fifth Korean National Health and Nutrition Examination Survey. Eur. J. Clin. Nutr. 2014, 69, 501–504. https://doi.org/10.1038/ejcn.2014.247.
- Navarro, A.M.; Abasheva, D.; Martínez-González, M.Á.; Ruiz-Estigarribia, L.; Martín-Calvo, N.; Sánchez-Villegas, A.; Toledo, E. Coffee Consumption and the Risk of Depression in a Middle-Aged Cohort: The SUN Project. Nutrients 2018, 10, 1333. https://doi.org/10.3390/nu10091333.
- Lucas, M.; Mirzaei, F.; Pan, A.; Okereke, O.I.; Willett, W.C.; O’Reilly, É.J.; Koenen, K.; Ascherio, A. Coffee, caffeine, and risk of depression among women. Arch. Intern. Med. 2011, 171, 1571–1578.
- Liu, Q.S.; Deng, R.; Fan, Y.; Li, K.; Meng, F.; Li, X.; Liu, R. Low dose of caffeine enhances the efficacy of antidepressants in major depressive disorder and the underlying neural substrates. Mol. Nutr. Food Res. 2017, 61, 1600910. https://doi.org/10.1002/mnfr.201600910.
- Min, J.; Cao, Z.; Cui, L.; Li, F.; Lu, Z.; Hou, Y.; Yang, H.; Wang, X.; Xu, C. The association between coffee consumption and risk of incident depression and anxiety: Exploring the benefits of moderate intake. Psychiatry Res. 2023, 326, 115307. https://doi.org/10.1016/j.psychres.2023.115307.
- Giuseppe, G.; Agnieszka, M.; Sabrina, C.; Andzrej, P.; Fabio, G. Coffee, tea, caffeine and risk of depression: A systematic review and dose-response meta-analysis of observational studies. Mol. Nutr. Food Res. 2015, 60, 223-234. https://doi.org/10.1002/mnfr.201500620.
- Wang, Y.; Wang, Z.; Gui, P.; Zhang, B.; Xie, Y. Coffee and caffeine intake and depression in postpartum women: A cross-sectional study from the National Health and Nutrition Examination Survey 2007–2018. Front. Psychol. 2023, 14, 1134522. https://doi.org/10.3389/fpsyg.2023.1134522.
- Makki, N.M.; Alharbi, S.T.; Alharbi, A.M.; Alsharif, A.S.; Aljabri, A.M. Caffeine Consumption and Depression, Anxiety, and Stress Levels Among University Students in Medina: A Cross-Sectional Study. Cureus 2023, 15, 48018. https://doi.org/10.7759/cureus.48018.
- Coelho, J.; Alves, P.; Canas, P.; Valadas, J.; Shmidt, T.; Batalha, V.; Ferreira, D.; Ribeiro, J.; Bader, M.; Cunha, R.; et al. Overexpression of Adenosine A2A Receptors in Rats: Effects on Depression, Locomotion, and Anxiety. Front. Psychiatry 2014, 5, 67. https://doi.org/10.3389/fpsyt.2014.00067.
- El Yacoubi, M.; Costentin, J.; Vaugeois, J. Adenosine A2A receptors and depression. Neurology 2003, 61, S82–S87. https://doi.org/10.1212/01.wnl.0000095220.87550.f6.
- Xinyi, G.; Shuyi, Z.; Weini, M.; Qixue, W.; Ying, L.; Chenyi, X.; Ying, X.; Ting, Z.; Li, Y.; Mingmei, Z. The Impact of Instant Coffee and Decaffeinated Coffee on the Gut Microbiota and Depression-Like Behaviors of Sleep-Deprived Rats. Front. Microbiol. 2022, 13, 778512. https://doi.org/10.3389/fmicb.2022.778512.
- Ruicheng, Z.; Lei, Z.; Wenqi, D.; Jiao, T.; Long, Y.; Deqin, G.; Yanbo, C. Caffeine alleviate lipopolysaccharide-induced neuroinflammation and depression through regulating p-AKT and NF-κB. Neurosci. Lett. 2024, 837, 137923. https://doi.org/10.1016/j.neulet.2024.137923.
- Pravin Popatrao, K.; Veeranjaneyulu, A. Augmentation of antidepressant effects of duloxetine and bupropion by caffeine in mice. Pharmacol. Biochem. Behav. 2014, 124, 238–244. https://doi.org/10.1016/j.pbb.2014.06.005.
- Cornelis, M.; Byrne, E.; Esko, T.; Nalls, M.; Ganna, A.; Paynter, N.; Monda, K.; Amin, N.; Fischer, K.; Renstrom, F.; et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol. Psychiatry 2015, 20, 647–656. https://doi.org/10.1038/mp.2014.107.





